When Condensation Occurs Does Water Vapor Absorb or Release Heat
Answered 5 years ago Author has 12K answers and 18M answer views. Water vapor can be produced from the evaporation or boiling of liquid water or from the sublimation of ice.

Condensation And The Water Cycle U S Geological Survey
14 How evaporation and condensation leads to the formation of rain.
. If the air should cool to the dew point temperature or below condensation may take place describe the Coriolis Effect and its influence in both hemispheres. As condensation occurs and liquid water forms from the vapor the water molecules become more organized and heat is released into the atmosphere as a result. For water to evaporate it must absorb heat from its surroundings.
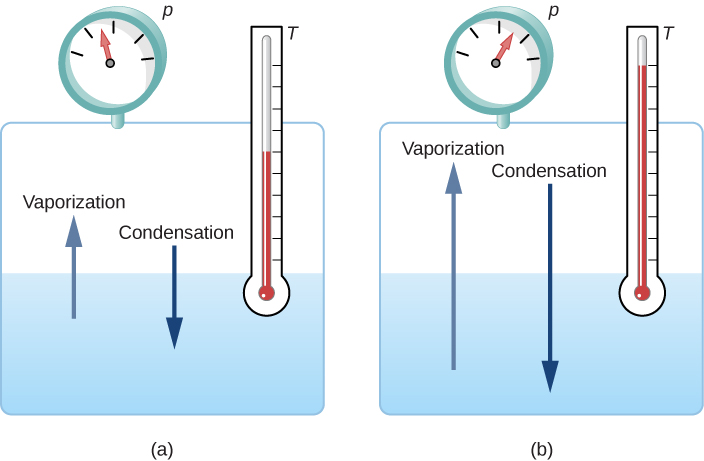
Energy released during condensation is the latent heat of condensation. The molar heat of vaporization is the heat absorbed by one mole of that substance. In the same way as the hotplate plate actively transfers heat of vaporization to the water the cold glass plate also actively absorbs heat of condensation.
The heat of condensation is numerically exactly equal to the heat vaporization but has the opposite sign. When 1 mol of water vapor at 100C condenses 407 kJ of heat are released into the surroundings. An exothermic reaction gives off heat energy.
Why does condensation release heat. As in jet condensers the cooling water is sprayed on the exhaust steam and there is direct contact between the exhaust steam and cooling water. If for example the glass plate had the same temperature of 100 C as the water vapor then no more water would condense in this case either since there would be no heat flow from the vapor to the glass ie.
When water vapor condenses to liquid water is heat absorbed or released quizlet. As evaporation occurs the temperature of the remaining liquid decreases. Freezing and condensation are also generally exothermic.
It is one state of water within the hydrosphere. 15 What are the 8 stages of the water cycle. 14 What is relative humidity quizlet.
This is the heat that was absorbed when the water was originally evaporated from the surface of the Earth a process which keeps the Earths surface climate much cooler that it would otherwise be if there were no water. Condensation is the change of state from a gas to a liquid. When warm air is cooled it looses its capacity to hold water vapor and if it cools enough it will begin to condensate.
Heat is released when vapor condenses into water and heat is absorbed to turn it into steam from water. Does deposition absorb heat or release heat. Deposition is exothermic--it releases heat.
15 When salt is introduced to water the temperature at which freezing occurs is. When condensation occurs does water vapor absorb or release heat Releas Does rising air warm or cool and does it do so by expansion or compression Cool by expansion In the early morning is relative humidity generally highest or lowest Highest continental polar Cold and dry Maritime polar Cool and humid Continental Tropical Hot and dry. Direct contact condensation DCC occurs when vapor is brought into contact with a cold liquid.
Sweat for instance absorbs heat from your skin in order to evaporate thus cooling your body. This typically occurs when water vapor molecules come into contact with cooler molecules. As a result the liquid molecules that remain now have lower kinetic energy.
Liquid molecules that have this certain threshold kinetic energy escape the surface and become vapor. Some content may have restrictions. Warmer air can hold more water vapor than cooler air.
As a gas condenses to a liquid heat is released. Why does condensation release heat. Condensation problems are most likely to occur in climates where temperatures frequently dip to 35F or colder over an extended period of timeWhat causes co.
The molar heat of condensation of a substance is the heat released by one mole of that substance as it is converted from a gas to a liquid. Under typical atmospheric conditions water vapor is continuously generated. It is called the Heat of Vaporization and works both ways.
Does melting absorb or release energy. This typically occurs when water vapor molecules come into contact with cooler molecules. This causes the water vapor molecules to lose some energy as heat.
When Does Condensation FormWhen the air temperature drops below its dew point excess moisture will be released in the form of condensation. An exothermic processes involves a negative change in enthalpy or a loss of heat. So in thinking about the fact that heat must be added to water to convert it to vapor then it holds that condensing the same water vapor back to liquid water would release heat back to the surrounding environment.
Since vaporization and condensation of a. As water vapor condenses into liquid it loses. 13 When condensation occurs does water vapor absorb or release heat.
Condensation is the process by which water vapor turns into liquid water. The process of condensation is very fast and efficient but here cooling water and condensed steam are mixed up. Once enough energy is lost the water vapor changes state into liquid.
707 views View upvotes Matthew Sullivan Still vaguely a physicist. In the case of evaporation the energy is absorbed by the substance whereas in condensation heat is released by the substance. 16 What are the 6 processes of the water cycle.
Water vapor water vapour or aqueous vapor is the gaseous phase of water. Water vapor is transparent like most constituents of the atmosphere. In the case of evaporation the energy is absorbed by the substance whereas in condensation heat is released by the substance.
12 When steam condenses what is happening to the heat and water molecules. Condensation is theprocess by which water vapor turns into liquid water. The heat removed from the surface through evaporation is thereby released again higher up in the atmosphere when clouds form.
The molar heat of vaporization of a substance is the heat absorbed by one mole of that substance as it is converted from a liquid to a gas. For example as moist air is lifted and cooled water vapor eventually condenses which then allows for huge amounts of latent heat energy to be released feeding the storm.
What Is The Process Of Changing Water Into Water Vapour By Heating Called Quora

Condensation National Geographic Society
What Is The Water Cycle Nasa Climate Kids
Water Cycle Understanding Global Change
Phase Changes University Physics Volume 2

Humidity Evaporation And Boiling Physics

Specific Heat Heat Of Vaporization And Density Of Water Article Khan Academy
Chapter 4 Water Vapor Atmospheric Processes And Phenomenon

Condensation Phase Change Britannica

Fresh Water Production From Atmospheric Air Technology And Innovation Outlook Sciencedirect
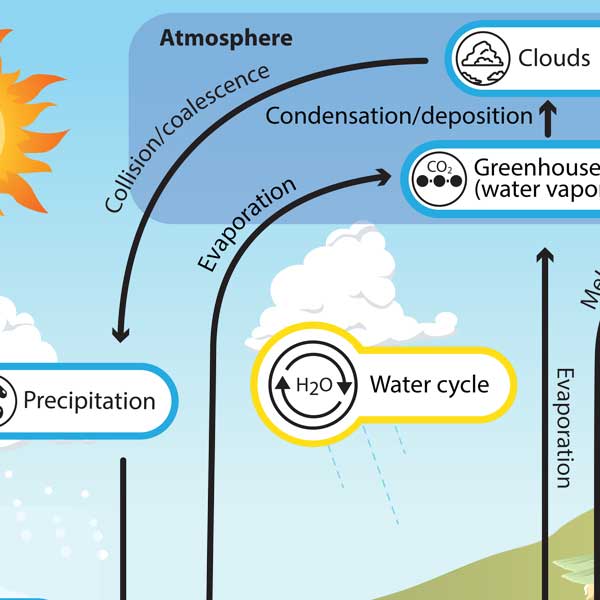
Water Cycle Understanding Global Change

Condensation And The Water Cycle U S Geological Survey
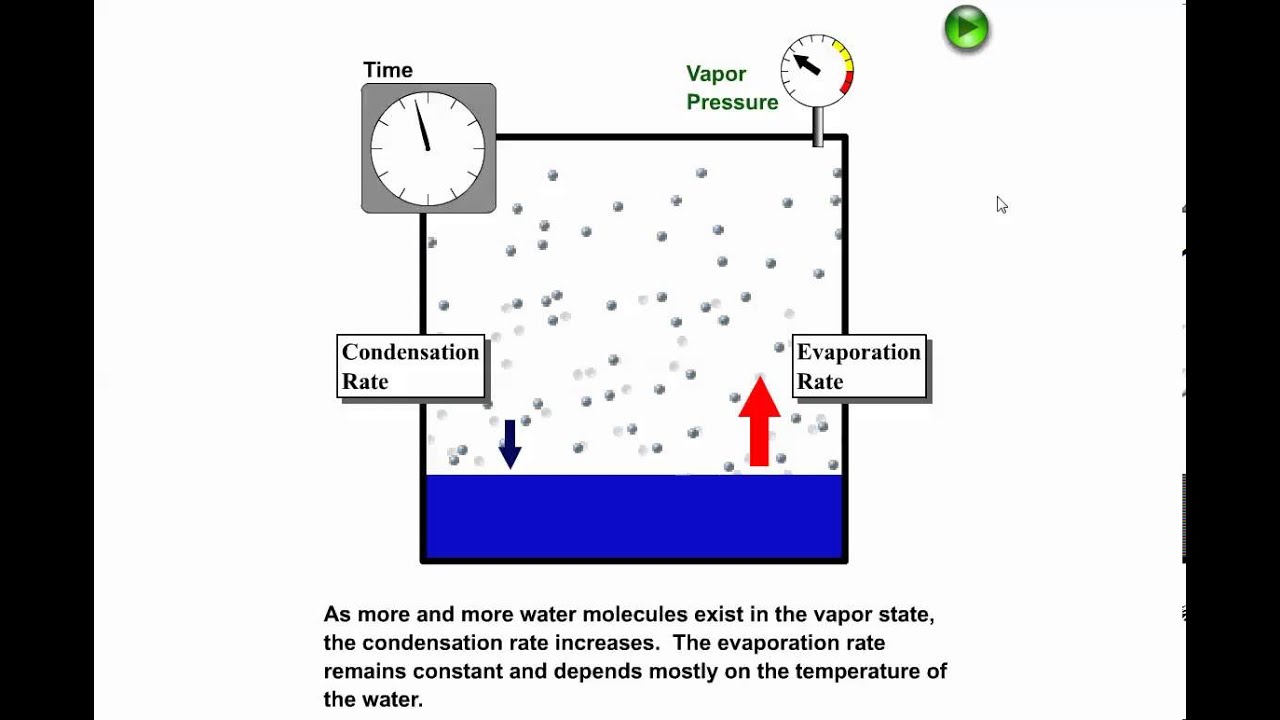
Phase Changes Boundless Chemistry

Exothermic Gives Off Heat Or Endothermic Absorbs Heat Chemistry Lessons Chemistry Gcse Chemistry

Water Vapor An Overview Sciencedirect Topics

When Condensation Occurs Does Water Vapor Absorb Or Release Heat Lisbdnet Com
Is Heat Absorbed Or Released When Water Vapor Condenses Quora

Take The Fog Out Of Thermal Windows Seattle Home Inspection Home Inspection Thermal Windows Seattle Homes

Comments
Post a Comment